| home |
| people |
| movies |
| publications |
| confocal |
| protocols |
| slideshow |
How to find us:
OHSU
School of Medicine
Department of Cell, Developmental & Cancer Biology
3181 SW Sam Jackson Park Rd
Basic Sciences L215
Portland, OR 97239
Research |
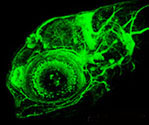
In vertebrates, placodes are transient epithelial thickenings (Figure 1) within the nonneural ectoderm that give rise to sensory neurons of the cranial ganglia (Figure 2, arrowheads) as well as the sensory structures of the nose and ear. The neurogenic placodes include the trigeminal placode that forms neurons of the trigeminal ganglia; the epibranchial (EB) placodes that generate the sensory neurons of the facial, glossopharyngeal, and vagal ganglia; and lateral line placodes that give rise to the lateral line ganglia and mechanosensory neuromasts in aquatic vertebrates. EB neurons innervate internal organs to transmit information such as heart rate, blood pressure, and visceral distension from the periphery to the CNS. Figure 1. Transverse section through the
cranial placode. Cranial placodes develop in a close
proximity with other embryonic tissues, including the neural tube,
neural crest, head mesoderm, and endoderm.
In our lab, we are interested in defining the cellular mechanisms of EB placode formation (see placode formation movie) and uncovering roles of various signaling pathways during early placode development (reverse genetic approach). In addition to a candidate gene approach, we are conducting a mutagenesis screen to find genes necessary for EB placode and ganglia development (forward genetic approach). We have already isolated a number of mutants defective in EB placode and ganglia development and efforts are underway to find mutated genes.
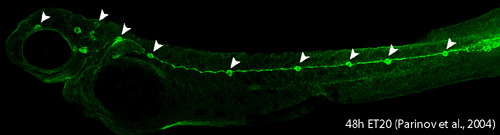
II. Development of lateral line system Lateral line system in aquatic vertebrates consists of mechanosensory organs called neuromasts (NM) (Figure 3, arrowheads) and lateral line nerves that innervate them. Each NM is a volcano-shaped structure with mechanosensory hair cells projecting microtubule-containing kinocilia and actin-based stereocilia through a central pore (Movie 1). Hair cells are surrounded by basally-located support cells. Since lateral line sensory cells are on the surface of the body, they are readily accessible for visualization and manipulation. The lateral line system is important for various behaviors, such as feeding, schooling, obstacle avoidance, and prey detection.
Figure 3. ET20 transgenic zebrafish embryo at 2 days post-fertilization. GFP reporter is expressed in a subset of support cells in each NM (arrowheads). The zebrafish lateral line is emerging as an excellent system to understand basic developmental events such as collective cell migration (see lateral line primordium migration movie) , proliferation and differentiation as well as mechanosensory hair cell development and regeneration in a relatively simple vertebrate system. Movie 1. This confocal movie was obtained from a 4-day old ET20 transgenic embryo that also carried an alpha-tubulin:dTomato transgene (a gift from T. Schilling lab). This transgene allows visualization of mechanosensory hair cells (center, red).
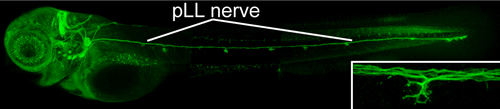
Within individual neurons, various types of cargo must be actively transported to specific compartments. For example, in the axon, synaptic proteins are targeted to axon terminals, while growth factors are often transported back to the cell body. This is accomplished primarily by microtubule-based transport mediated by two types of molecular motors, kinesins and dyneins, that respectively move cargo away from and towards the cell body. Various types of vesicles and organelles are moved by fast transport both in anterograde and retrograde directions at speeds of 0.5-10 μm/sec. Zebrafish are an excellent vertebrate system for genetic analyses of microtubule-based transport during nervous system development. Zebrafish embryos and larvae remain transparent throughout development, and there are established techniques to express and visualize fluorescently-tagged proteins in specific cell populations. In particular, the posterior lateral line (pLL) nerve is ideal for studying axon transport and microtubule dynamics in vivo: it contains a small number of long sensory axons that run along the surface of the body wall to make stereotyped connections with mechanosensory hair cells (Figure. 4).
Figure 4. Posterior lateral line nerve is used to study axonal transport in zebrafish. Inset shows axon terminals that innervate invidual sensory organ (neuromast). Because of its superficial positioning, the pLL can be easily imaged without invasive techniques. We have developed methods to visualize axonal transport in live zebrafish embryos and larva. Constructs encoding fluorescently tagged proteins under the control of the neural promoter are injected into zebrafish zygotes. This results in mosaic expression of the tagged proteins in the developing pLL ganglion. Live embryos with pLL neuron expression are then mounted in low-melting-point agarose and imaged using a confocal microscope fitted with a 60X/NA=1.2 water immersion lens. Labeled puncta (representing discrete cargo) are imaged 3 to 5 times per second over 3-6 min intervals at proximal and distal positions of the pLL nerve (Movie 2).
Movie 2. Lamp1-mTangerine labeled lysosomes are transported in the pLL nerve. Wildtype zebrafish expressing Lamp1-mTangerine in 2 axons of the pLL nerve at 3 dpf. Anterograde transport is from left to right. Scale bar=10 µm. Time is in seconds. We also developed protocol for dual cargo imaging. Movie 3 shows co-transport of Jip3-mCherry and Lamp1-EGFP.
Movie 3. Lysosomes are co-transported with Jip3 in pLL axons. Sequential imaging of Jip3-mCherry and Lamp1-EGFP in a pLL axon of a wildtype 3 dpf zebrafish larva. Pink and yellow arrowheads denote two individual lysosomes moving in the retrograde direction that are positive for Jip3. The green arrowhead indicates a Lamp1-positive lysosome moving in the anterograde direction that is not labeled by Jip3. Merge (top), Lamp1-EGFP only (middle) and Jip3-mCherry (bottom) panels are shown. Scale bar is 10 μm. Time is in seconds. |
Last update: April 2021
home I research I people I movies I publications I confocal I positions I links
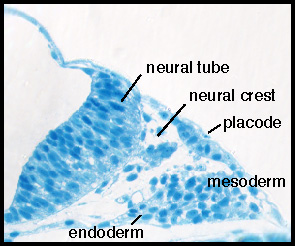
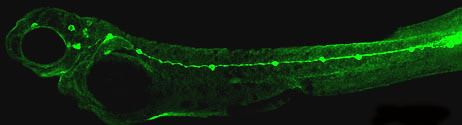
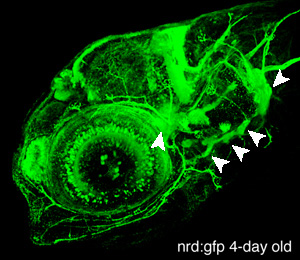 Figure 2. This confocal projection was obtained from a 4-day old neurod:gfp transgenic zebrafish. Neurod is a proneural transcription factor expressed in differentiating neurons of the CNS and peripheral nervous system (olfactory neurons, epiphysis, cranial ganglia, retina, etc.). The gfp expression also allows visualization of the central and peripheral neuronal projections. We use this zebrafish strain to conduct mutagenesis screens and characterize various mutants defective in cranial ganglia (arrowheads) development.
Figure 2. This confocal projection was obtained from a 4-day old neurod:gfp transgenic zebrafish. Neurod is a proneural transcription factor expressed in differentiating neurons of the CNS and peripheral nervous system (olfactory neurons, epiphysis, cranial ganglia, retina, etc.). The gfp expression also allows visualization of the central and peripheral neuronal projections. We use this zebrafish strain to conduct mutagenesis screens and characterize various mutants defective in cranial ganglia (arrowheads) development.